Only 10 $ per sample for interpreting of your FT-IR, Raman, XRD, UV-Vis, and XPS spectrum
Payment Upon Completion
Send your spectra…
In the realm of chemistry, the concept of allotropy unveils the mesmerizing ability of an element to exist in multiple forms, known as allotropes, each exhibiting distinct physical and chemical properties. Among the myriad elements that showcase this intriguing phenomenon, carbon stands out as a versatile and captivating element with a plethora of allotropes. Understanding the diverse carbon allotropes, their mechanical and chemical properties, as well as the characterization methods used to unveil their secrets, is essential for unlocking their potential in various scientific and technological applications.
Carbon Allotropes: A Kaleidoscope of Structures and Properties
Carbon, with its ability to form strong covalent bonds and diverse molecular structures, manifests in several allotropes, each with unique properties and applications. Here are some of the prominent carbon allotropes:
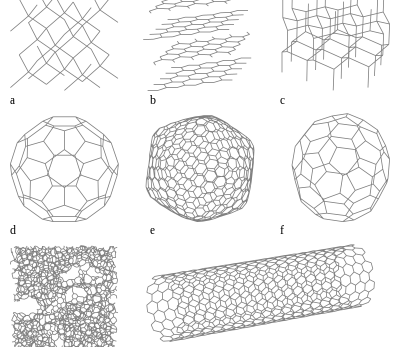
1. Diamond: The epitome of elegance and durability, diamond features a three-dimensional network of carbon atoms arranged in a tetrahedral structure. Renowned for its exceptional hardness, thermal conductivity, and optical properties, diamond finds applications in jewellery, cutting tools, and industrial abrasives.
2. Graphite: In contrast to diamond’s rigid structure, graphite embodies layers of carbon atoms arranged in hexagonal rings, imparting lubricating properties. Graphite is commonly used in pencil leads, lubricants, and electrodes due to its soft and slippery nature.
3. Graphene: A single layer of graphite arranged in a two-dimensional hexagonal lattice structure, graphene boasts remarkable mechanical strength, electrical conductivity, and thermal properties. This wonder material holds promise for applications in electronics, energy storage, and sensors.
4. Carbon Nanotubes: These cylindrical structures composed of rolled-up graphene sheets exhibit exceptional mechanical strength, electrical conductivity, and thermal properties. Carbon nanotubes find applications in nanotechnology, composites, and electronics due to their unique structural characteristics.
5. Fullerenes: Hollow carbon molecules with cage-like structures, fullerenes like Buckminsterfullerene (C60) possess intriguing properties such as high electron affinity and reactivity. Fullerenes are utilized in diverse fields ranging from drug delivery to superconductors.
Mechanical and Chemical Properties of Carbon Allotropes
Each carbon allotrope showcases a distinctive set of mechanical and chemical properties based on its unique structure and bonding arrangement:
– Diamond: Exceptional hardness, transparency, high thermal conductivity.
– Graphite: Softness, lubricating properties, opaque nature.
– Graphene: High electrical conductivity, mechanical strength, thermal conductivity.
– Carbon Nanotubes: Exceptional mechanical strength, electrical conductivity, and thermal properties.
– Fullerenes: High electron affinity, reactivity, unique cage-like structures.
Characterization Methods for Carbon Allotropes
To unravel the mysteries of carbon allotropes and understand their properties at a molecular level, various sophisticated characterization techniques are employed:
1. Fourier Transform Infrared Spectroscopy (FTIR)

Fourier-transform infrared (FTIR) spectroscopy is another powerful analytical technique that can aid in the characterization of different allotropies of carbon by providing information about their chemical bonding, functional groups, and structural properties. Here’s how FTIR analysis can be utilized to study various carbon allotropes:
a. Functional Group Identification: FTIR spectroscopy can be used to identify specific functional groups present in different carbon allotropes based on the characteristic absorption bands observed in their infrared spectra. For example, the presence of sp2 and sp3 hybridized carbon bonds in graphene, carbon nanotubes, and diamond can be distinguished by analyzing the peaks corresponding to C=C and C-H stretching vibrations, respectively. Additionally, functional groups such as hydroxyl (-OH), carbonyl (C=O), carboxyl (-COOH), and epoxy (-O-) groups can be detected in carbon materials through their distinctive IR absorption bands, allowing researchers to assess the surface chemistry and reactivity of the allotropes.
b. Structural Analysis: FTIR spectroscopy can provide insights into the structural characteristics of carbon allotropes by probing the vibrational modes of carbon-carbon bonds and other chemical interactions within the materials. The presence of sp2 and sp3 hybridized carbon atoms, aromatic rings, double bonds, and functional groups can be inferred from the intensity, position, and shape of the absorption bands in the FTIR spectrum. By correlating the vibrational frequencies of carbon allotropes with their structural features, researchers can elucidate the bonding configurations, lattice arrangements, and crystallographic orientations of the materials.
c. Surface Modification and Functionalization: FTIR spectroscopy is a valuable tool for studying surface modifications, functionalization reactions, and chemical interactions on the surface of carbon allotropes. By comparing the FTIR spectra of pristine and modified carbon samples, researchers can identify changes in the absorption bands associated with functional groups introduced during surface treatments, chemical derivatization, or doping processes. This enables the characterization of surface functionalization strategies, quantification of surface coverage, and evaluation of chemical stability in functionalized carbon materials.
d. Quantitative Analysis: FTIR spectroscopy can be utilized for quantitative analysis of functional groups, impurities, and contaminants in carbon allotropes by measuring the absorbance intensities at specific wavenumbers corresponding to characteristic vibrational modes. By establishing calibration curves or using peak area integration methods, researchers can quantify the relative concentrations of different functional groups or impurities in a carbon sample, providing valuable information about its chemical composition, purity, and quality.
e. Stability and Degradation Studies: FTIR spectroscopy can be employed to investigate the stability, degradation mechanisms, and chemical reactivity of carbon allotropes under various environmental conditions. By monitoring changes in the FTIR spectra over time or upon exposure to external factors (e.g., temperature, humidity, oxidation), researchers can assess the material’s resistance to degradation, identify degradation products or by-products, and elucidate the underlying chemical processes that influence its performance and longevity.
2. Raman Spectroscopy
By studying the vibrational modes of carbon materials, Raman spectroscopy offers valuable information about their structural properties and defects. Raman spectroscopy is a powerful analytical technique that can provide valuable insights into the structural and vibrational properties of different carbon allotropes. Here’s how Raman spectroscopy can help characterize various carbon allotropes:
a. Structural Analysis: Raman spectroscopy can distinguish between different carbon allotropes based on their unique structural characteristics. Each allotrope exhibits specific Raman-active vibrational modes, allowing researchers to identify and differentiate between diamond, graphite, graphene, carbon nanotubes, and fullerenes.
b. Defect Detection: Carbon allotropes may contain defects or impurities that can influence their properties. Raman spectroscopy can detect and characterize these defects by analyzing changes in the Raman spectra, such as shifts in peak positions or intensity variations. This information is crucial for understanding the quality and purity of carbon materials.
c. Quantitative Analysis: Raman spectroscopy can be used for quantitative analysis of carbon allotropes, providing information about the relative abundance of different phases or structures within a sample. By correlating Raman spectral features with specific carbon allotropes, researchers can quantitatively assess the composition and distribution of various forms of carbon in a sample.
d. Chemical Functionalization: Raman spectroscopy is sensitive to chemical modifications and functional groups present on the surface of carbon allotropes. By analyzing changes in Raman spectra upon functionalization or chemical treatment, researchers can characterize the interaction between carbon materials and other substances, enabling the design of tailored functionalized carbon materials for specific applications.
3. X-ray Photoelectron Spectroscopy (XPS)
XPS is another valuable technique that can aid in the characterization of different allotropies of carbon. Here’s how XPS analysis can provide insights into the structural and chemical properties of various carbon allotropes:
a. Elemental Composition: XPS analysis can determine the elemental composition of carbon allotropes by measuring the binding energies of core-level electrons, such as the carbon 1s peak. Different carbon allotropes exhibit distinct binding energy values for their core-level electrons due to variations in the local chemical environment and bonding configurations. By comparing the XPS spectra of carbon allotropes with reference data, researchers can identify the presence of specific elements and quantify their relative concentrations.
b. Chemical State Analysis: XPS analysis can reveal information about the chemical state and bonding characteristics of carbon allotropes. The peak shapes, positions, and intensities in the XPS spectra provide insights into the oxidation state, functional groups, and bonding configurations present in a carbon sample. For example, XPS can differentiate between sp2 and sp3 hybridized carbon atoms in graphene and diamond, respectively, based on their distinct chemical environments and electronic structures.
c. Surface Sensitivity: XPS analysis is a surface-sensitive technique that probes the top few nanometers of a material, making it well-suited for characterizing the surface chemistry of carbon allotropes. By analyzing the elemental composition and chemical states at the surface of a carbon sample, researchers can gain valuable information about surface contaminants, functionalization, and modifications that may influence the material’s properties and reactivity.
d. Dopant Identification: XPS analysis can help identify dopants or impurities incorporated into carbon allotropes to modify their electronic, optical, or catalytic properties. By analyzing the XPS spectra of doped carbon materials, researchers can detect changes in the core-level binding energies and chemical states of the dopant atoms, providing insights into their distribution, concentration, and interaction with the host carbon lattice.
e. Depth Profiling: XPS analysis can also be combined with depth profiling techniques to investigate the chemical composition and structure of carbon allotropes as a function of depth below the surface. Depth profiling methods, such as angle-resolved XPS or sputter depth profiling, allow researchers to study the layer-by-layer composition, doping profiles, and interface properties of carbon materials, enabling a comprehensive understanding of their structure-property relationships.
4. Ultraviolet-Visible Spectroscopy (UV-Vis)
UV-Vis spectroscopy aids in studying the optical properties of carbon allotropes, including absorption and emission spectra.
UV-Vis spectroscopy is another valuable technique that can aid in the characterization of different allotropies of carbon by providing insights into their electronic and optical properties. Here’s how UV-Vis analysis can be utilized to study various carbon allotropes:
a. Bandgap Determination: UV-Vis spectroscopy can be used to determine the bandgap energy of carbon allotropes, which is a crucial parameter that influences their electronic and optical properties. By measuring the absorption spectrum of a carbon sample in the UV and visible regions, researchers can identify the onset of absorption (i.e., the bandgap energy) and characterize the material’s semiconducting or insulating behavior. Different carbon allotropes, such as graphene, carbon nanotubes, and diamond, exhibit distinct bandgap energies due to variations in their electronic structure and bonding configurations.
b. Optical Absorption Features: UV-Vis spectroscopy can reveal information about the optical absorption features of carbon allotropes, such as excitonic transitions, interband transitions, and localized electronic states. The absorption spectrum of a carbon sample can exhibit characteristic peaks, shoulders, or broad absorption bands corresponding to specific electronic transitions within the material. By analyzing the shape, intensity, and position of these absorption features, researchers can gain insights into the electronic structure, energy levels, and optical properties of different carbon allotropes.
c. Defects and Functional Groups: UV-Vis spectroscopy can be used to detect defects, functional groups, and chemical modifications in carbon allotropes that affect their electronic and optical properties. Defect-induced states, surface functionalization, and doping can introduce additional absorption features or modify the intensity of existing peaks in the UV-Vis spectrum of a carbon sample. By comparing the UV-Vis spectra of pristine and modified carbon materials, researchers can identify changes in the electronic structure, bandgap energy, and optical response resulting from defects or functionalization.
d. Quantitative Analysis: UV-Vis spectroscopy can also be employed for quantitative analysis of carbon allotropes by correlating the absorption intensity with the concentration of specific components or impurities in a sample. By measuring the absorbance at characteristic wavelengths and establishing calibration curves for different carbon species or dopants, researchers can quantify the relative abundance of components in a complex mixture or determine the doping level in doped carbon materials.
e. Stability and Degradation Studies: UV-Vis spectroscopy can provide valuable information about the stability, degradation, and photochemical behavior of carbon allotropes under various environmental conditions. By monitoring changes in the UV-Vis absorption spectrum over time or under different exposure conditions (e.g., light irradiation, temperature variations), researchers can assess the material’s photochemical stability, degradation mechanisms, and resistance to environmental factors that may impact its performance and longevity.
5. X-ray Diffraction (XRD)
X-ray diffraction (XRD) analysis is another powerful technique that can provide valuable insights into the structural properties of different carbon allotropes. Here’s how XRD analysis can help characterize various allotropies of carbon:
a. Crystal Structure Determination: XRD analysis can be used to determine the crystal structure of carbon allotropes by analyzing the diffraction patterns generated when X-rays interact with the periodic arrangement of atoms in a material. Different carbon allotropes have distinct crystal structures, such as the hexagonal lattice of graphite, the cubic structure of diamond, and the helical structure of carbon nanotubes. By comparing experimental XRD patterns with reference data, researchers can identify and confirm the crystal structure of a carbon allotrope.
b. Phase Identification: XRD analysis can help identify and distinguish between different phases or polymorphs of carbon allotropes present in a sample. By analyzing the positions and intensities of diffraction peaks in the XRD pattern, researchers can determine the presence of specific allotropes, such as graphite, diamond, graphene, carbon nanotubes, and fullerenes. This information is essential for characterizing the composition and phase distribution within a carbon sample.
c. Crystallite Size and Orientation: XRD analysis can provide information about the crystallite size and orientation of carbon allotropes. By analyzing the broadening of XRD peaks, researchers can estimate the average crystallite size of a material, which is crucial for understanding its structural properties. Additionally, XRD can reveal information about the preferred orientation or texture of crystallites within a sample, offering insights into the growth and alignment of carbon allotropes.
d. Strain Analysis: XRD analysis can also be used to investigate the presence of strain or defects in carbon allotropes. Changes in the peak positions and peak shapes in the XRD pattern can indicate the presence of lattice strain, dislocations, or defects in the crystal structure of a material. By quantifying these structural imperfections, researchers can assess the mechanical stability and performance of carbon allotropes.
e. Thermal Stability and Phase Transitions: XRD analysis can be employed to study the thermal stability and phase transitions of carbon allotropes under varying temperature and pressure conditions. By monitoring changes in the XRD patterns as a function of temperature or pressure, researchers can identify phase transformations, melting points, and structural changes in carbon materials, providing crucial information for understanding their behaviour under different environmental conditions.
Conclusion
In conclusion, the captivating world of carbon allotropes unveils a treasure trove of possibilities for scientific exploration and technological innovation. By delving into the diverse structures and properties of carbon allotropes and employing advanced characterization methods, researchers can unlock the full potential of these fascinating materials across a wide range of applications. The allure of carbon allotropes continues to inspire groundbreaking discoveries and advancements in materials science and beyond.