Corrosion is a major concern in many industries, as it can cause significant damage to infrastructure and equipment. To prevent corrosion, it is important to monitor the corrosion rate and take appropriate measures to mitigate it.
Two common methods for corrosion monitoring are electrochemical impedance spectroscopy (EIS) and polarization methods. While both methods are used to measure the corrosion rate, they differ in their approach and the information they provide.
EIS measures the impedance of a material as a function of frequency. By analyzing the impedance spectrum, it is possible to determine the electrical properties of the material, such as its resistance, capacitance, and conductivity. EIS can be used to monitor corrosion by measuring changes in the impedance spectrum over time. Corrosion can cause changes in the electrical properties of a material, which can be detected by EIS.
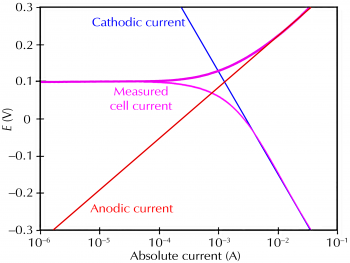
Polarization methods, on the other hand, measure the potential and current of a material under an applied voltage or current. There are two main types of polarization methods: potentiodynamic and potentiostatic. Potentiodynamic polarization measures the current as the potential is swept over a range of values, while potentiostatic polarization measures the potential as the current is held constant.
One of the main differences between EIS and polarization methods is their sensitivity to different types of corrosion. EIS is more sensitive to localized corrosion, such as pitting and crevice corrosion, while polarization methods are more sensitive to uniform corrosion. This is because localized corrosion can cause changes in the electrical properties of a material, which can be detected by EIS, while uniform corrosion does not typically cause such changes.
Another difference between EIS and polarization methods is their ability to provide information about the corrosion mechanism. EIS can provide information about the electrochemical reactions that occur during corrosion, such as the formation of passive films and the dissolution of metal ions. Polarization methods, on the other hand, provide information about the kinetics of the corrosion reaction, such as the activation energy and the rate constant.
EIS and polarization methods also differ in their ease of use and cost. EIS requires specialized equipment and expertise to perform, while polarization methods can be performed with simpler equipment and require less expertise. However, EIS provides more detailed information about the corrosion mechanism and is more sensitive to localized corrosion, which can be important in certain applications.
In summary, both EIS and polarization methods are useful for corrosion monitoring, but they differ in their sensitivity to different types of corrosion, their ability to provide information about the corrosion mechanism, and their ease of use and cost. Choosing the appropriate method for a particular application depends on the specific corrosion concerns and the desired level of detail in the corrosion monitoring.